Even when collected simultaneously, fluid (blood/plasma) and imaging (PET/MRI) biomarkers do not necessarily reflect concurrent biological processes, as they capture fundamentally different aspects of Alzheimer's disease (AD) progression. Clara Sorensen is a graduate student in Dr Tosun's lab who uses AI for the discovery and validation of multi-modal biomarkers of neurodegenerative diseases including AD and Parkinson's disease (PD). She has been working on building computational biomarkers using graph neural networks to both predict the progression of AD pathology and to predict protein accumulation (Aβ and tau isoforms, glial fibrillary acidic protein (GFAP), and neurofilament light (NfL)) from multimodal image-based (structural MRI, diffusion MRI, and PET) graphs.
At AD/PD 2025 in Vienna, Austria, Clara will be presenting her work to better understand temporal changes in how plasma biomarkers reflect PET-detectable AD pathology. This work found that the associations between PET and plasma biomarkers evolved distinctly over time -- variance in plasma Aβ42/40 was better explained by future PET imaging (up to 4 years ahead), while plasma p-tau217 maintained consistently strong associations across all timeframes (plasma sampling 0-4 years ahead of PET). Similarly, p-tau biomarkers showed highest sensitivity for time-matched PET status, whereas Aβ42/40 sensitivity actually improved when predicting PET status 4 years post-plasma collection.
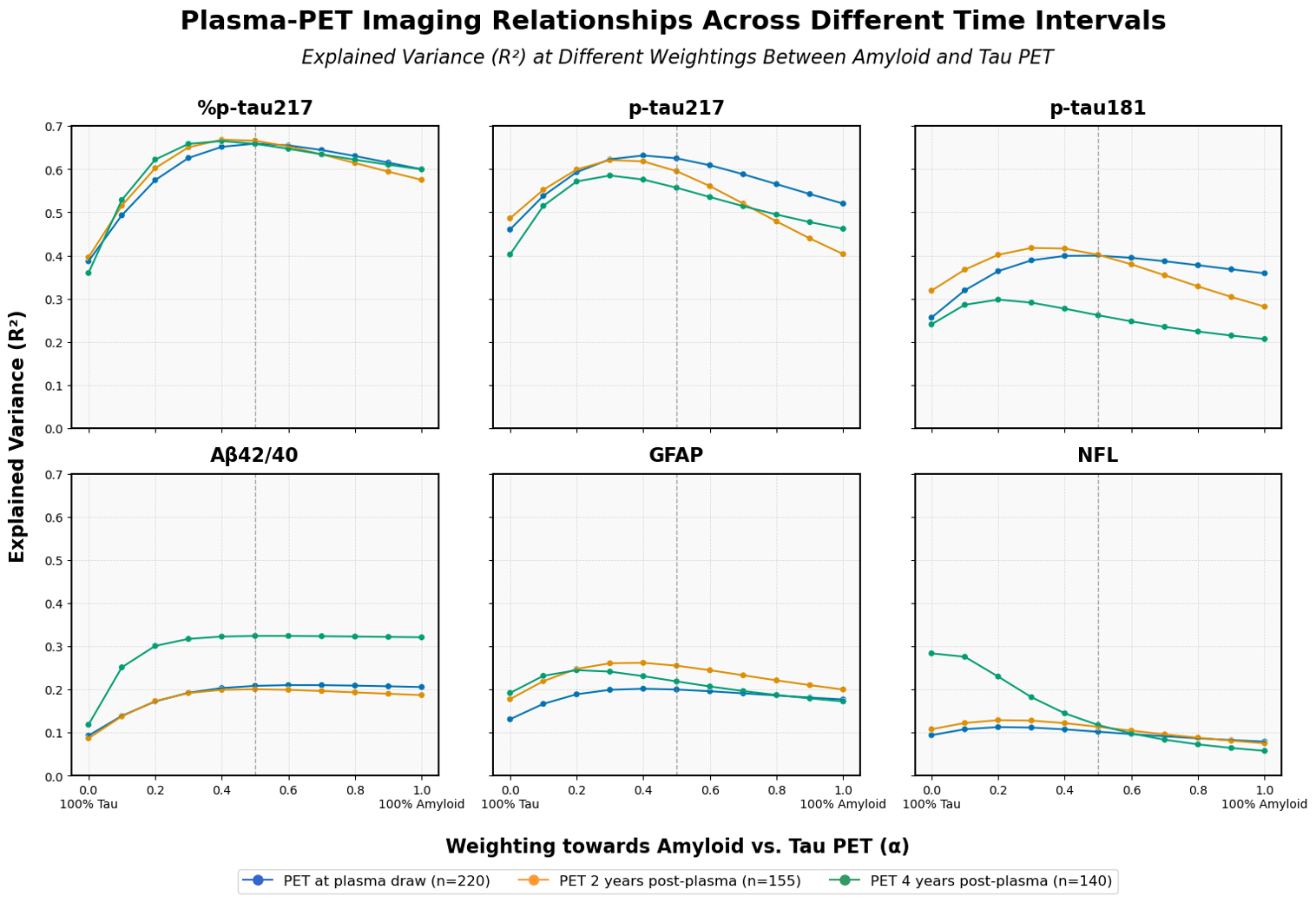
These findings suggest these biomarkers have different optimal screening timeframes across the AD...
Alzheimer’s disease (AD) is the predominant form of age-related dementia, and presents with multifactorial etiology. The accumulation of amyloid (Aβ) plaques, followed by tau tangles, ultimately contributes to synaptic dysfunction, atrophy, and eventually cognitive decline. The glymphatic system works to clear waste from the brain. Emerging evidence suggests that dysfunctional clearance of Aβ may exacerbate its aggregation and accelerate AD progression, particularly in the preclinical stages of the disease. The exact mechanism is unknown.
Serena Tang is a graduate student in Dr Tosun's lab who has been working on characterizing the role of glymphatic clearance in AD by designing computational tools to quantify perivascular spaces, the structural component of the glymphatic system. She is using statistical methods to unravel their relationship to drivers of glymphatic function and biomarkers of AD, namely cerebrovascular function and sleep. In leveraging large, multi-modal datasets (e.g. the Alzheimer’s Disease Neuroimaging Initiative (ADNI)) and experimental datasets to study these relationships across the AD continuum, her work will provide a better understanding of the brain’s waste clearance system to unravel the early pathophysiology of AD and unveil modifiable factors that can slow AD progression and preserve cognitive health.
Serena presented her work at two conferences in poster format, sharing results from an investigation of relationships between enlarged perivascular spaces (EPVS) as a measure of glymphatic clearance integrity, white matter hyperintensities (WMH) as a measure of cerebrovascular integrity, along with Aβ and APOE ε4 genotype as biomarkers for AD...